Revolutionary Device Set to Transform Lives of Individuals with Hearing Loss
OKLAHOMA CITY, OK – Hearts for Hearing, a leading local hearing healthcare center, has been selected as one of just seven investigational sites nationwide for Envoy Medical’s pivotal clinical trial of the revolutionary Acclaim® cochlear implant (Acclaim CI) – the first fully internal cochlear implant of its kind.
“Envoy Medical is thrilled to be working with Hearts for Hearing as one of the seven investigational sites involved in this pivotal clinical study. It was important to us to work with cochlear implant programs that have an amazing team of providers with a strong commitment to research and innovation – Hearts for Hearing checked all our boxes,” says Brent Lucas, CEO of Envoy Medical.
In a significant development, Hearts for Hearing will activate one of the first few devices, marking a historic milestone in hearing technology.
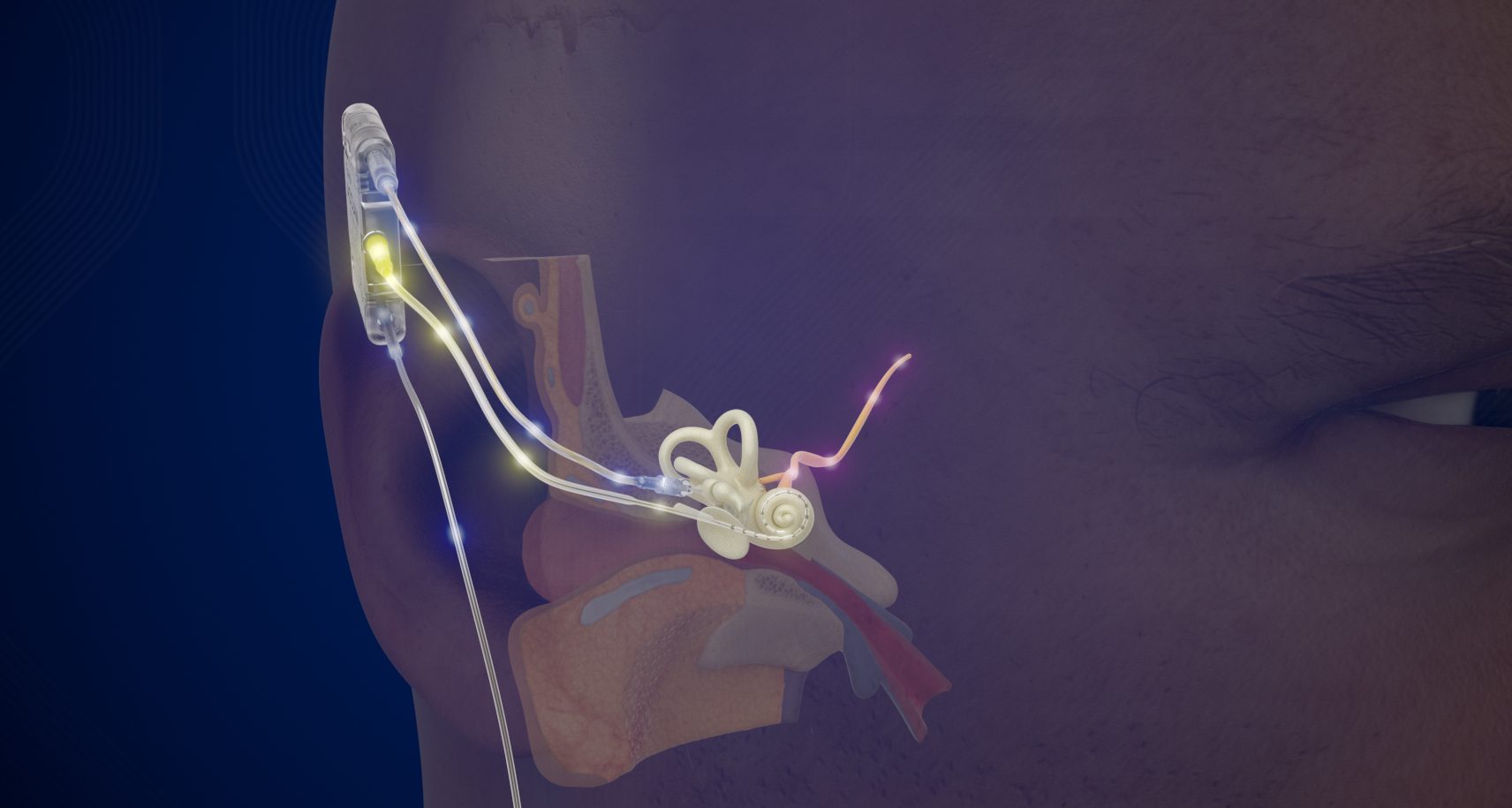
Unlike any existing cochlear implant, the Acclaim CI operates completely internally without an external processor. Instead of using a microphone, the device utilizes a specialized sensor that works with the ear’s natural anatomy to capture sound before converting it to electronic signals.
Dr. Wayne Berryhill of Oklahoma Otolaryngology, Principal Investigator and Otologist on the study, stated: “We are on the frontier of cochlear implant technology and expanding the horizons of individuals with hearing loss. This device can change the trajectory of the cochlear implant industry and give individuals with hearing loss new opportunities to connect with the people they love.”
With participation in this trial, Hearts for Hearing continues its legacy of improving the lives of individuals with hearing loss. Dr. Sara Neumann, the audiologist leading the study at Hearts for Hearing, expressed her enthusiasm: “I am excited to be at the forefront of cochlear implant innovations with the Envoy Acclaim. My hope is that completely implantable cochlear implant technology will encourage more individuals to seek treatment for their hearing loss.”
The first Acclaim CI device in Oklahoma, implanted earlier this month, is scheduled for activation in early May.
About the Acclaim CI: The Acclaim CI is a fully implanted cochlear implant that previously received Breakthrough Device Designation from the FDA. It is currently differentiated from other cochlear implants in that it is designed to: (1) be fully implanted with no external components attached to the side of the head, (2) leverage the natural anatomy of the ear to pick up sound rather than rely on an external or sub-dermal microphone, (3) allow for all day hearing, and (4) have a battery lasting multiple days between recharge.Additional study-specific updates can be found on ClinicalTrials.gov, or by contacting Envoy Medical. CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use
About Hearts for Hearing: Hearts for Hearing is a comprehensive hearing health care clinic for children and adults headquartered in Oklahoma City, with offices in Tulsa and Norman, and includes a state-of-the-art Mobile Care Clinic. The organization was founded in 2003 as a 501(c)3 to provide funding for the first set of hearing aids for children with hearing loss in Oklahoma. Its mission has expanded significantly to become one of the leading hearing health care centers in the country offering audiology, speech-language therapy, and leading-edge research in age ranges from newborn to senior adults. Learn more at www.heartsforhearing.org